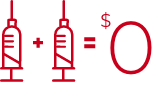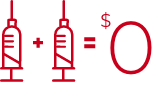Coverage & Coding
SHINGRIX has broad insurance coverage for your patients 50 years and older. 1,2, * For billing, use the codes listed below the coverage section.
Recommend with certainty:
SHINGRIX is now $0 for most patients 50 years and older. 1,2,†
For your patients with commercial insurance plans ‡ :
- Average copays of just $4 or less 1
- 98% of commercial patients pay $0 for SHINGRIX 1
For your patients with Medicare Part D :
Great coverage is now even better:
- All your Medicare Part D patients can receive SHINGRIX for $0 at their pharmacy. 2
* Coverage represents access to reimbursement from a health plan with restrictions appropriate to the Advisory Committee on Immunization Practices (ACIP) recommendation(s) and/or prescribing information. Veterans Affairs (VA) and Indian Health Service (IHS) lives have been omitted when calculating the percentage of lives. 1
† Coverage and cost may vary and are subject to change without notice. Reimbursement decisions are made by individual insurance plans.
‡ Based on IQVIA data of paid [2023] SHINGRIX claims.
Coding
Use the following codes when billing SHINGRIX:
CPT Code (Product):
90750
CPT Code (Administration)
1 vaccine administered:
Each additional vaccine administered during same encounter:
90471
90472
ICD-10-CM Code (Encounter for Immunization):
Z23
Administration Modifier for Medicare:
GY
MVX Code:
SKB
CVX Code:
187 §
§ The CDC also created CVX code 188 for "zoster vaccine, unspecified formulation" to facilitate transmission of administration data when the exact formulation of the vaccine is not known (such as when recording data from a vaccination card).
CDC=Centers for Disease Control and Prevention; CPT=Current Procedural Terminology; ICD-10-CM=International Classification of Diseases, 10th Revision, Clinical Modification; MVX=Manufacturers of Vaccines; CVX=vaccine administered.
How SHINGRIX is supplied
SHINGRIX is supplied as an outer package of 1 dose (NDC 58160-819-12) containing:
- Adjuvant Suspension Component (Vial 1 of 2) NDC 58160-829-01
- Lyophilized gE Antigen Component (Vial 2 of 2) NDC 58160-828-01
SHINGRIX is supplied as an outer package of 10 doses (NDC 58160-823-11) containing:
- Adjuvant Suspension Component (10 vials) NDC 58160-829-03
- Lyophilized gE Antigen Component (10 vials) NDC 58160-828-03
The GSK Vaccines Reimbursement Support Center is a resource for physicians, physician office staff, and pharmacists to help address questions about billing, coding, and reimbursement for GSK vaccines.
GSK Vaccines Reimbursement Support Center contact information:
Monday–Friday 8:00 am to 8:00 pm ET
You may also be interested in:
See how you and your staff can reconstitute SHINGRIX in 4 steps.
Learn how to store SHINGRIX and about the importance of proper dosing.
Talk to your patients about what to expect, including common adverse reactions.
Expand/Collapse
Indication & Important Safety Info
Indication
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
SHINGRIX is a vaccine indicated for prevention of herpes zoster (HZ) (shingles):
- in adults aged 50 years and older.
- in adults aged 18 years and older who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.
SHINGRIX is not indicated for prevention of primary varicella infection (chickenpox).
Important Safety Information
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- SHINGRIX is contraindicated in anyone with a history of a severe allergic reaction (eg, anaphylaxis) to any component of the vaccine or after a previous dose of SHINGRIX
- Review immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of SHINGRIX
- In a postmarketing observational study, an increased risk of Guillain-Barré syndrome was observed during the 42 days following vaccination with SHINGRIX
- Syncope (fainting) can be associated with the administration of injectable vaccines, including SHINGRIX. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope
- Solicited local adverse reactions reported in individuals aged 50 years and older were pain (78%), redness (38%), and swelling (26%)
- Solicited general adverse reactions reported in individuals aged 50 years and older were myalgia (45%), fatigue (45%), headache (38%), shivering (27%), fever (21%), and gastrointestinal symptoms (17%)
- Solicited local adverse reactions reported in autologous hematopoietic stem cell transplant recipients (aged 18 to 49 and ≥50 years of age) were pain (88% and 83%), redness (30% and 35%), and swelling (21% and 18%)
- Solicited general adverse reactions reported in autologous hematopoietic stem cell transplant recipients (aged 18 to 49 and ≥50 years of age) were fatigue (64% and 54%), myalgia (58% and 52%), headache (44% and 30%), gastrointestinal symptoms (21% and 28%), shivering (31% and 25%), and fever (28% and 18%)
- The data are insufficient to establish if there is vaccine-associated risk with SHINGRIX in pregnant women
- It is not known whether SHINGRIX is excreted in human milk. Data are not available to assess the effects of SHINGRIX on the breastfed infant or on milk production/excretion
- Vaccination with SHINGRIX may not result in protection of all vaccine recipients